Clean Room Assembly & Packaging
ISO-certified environments for sterile and sensitive products
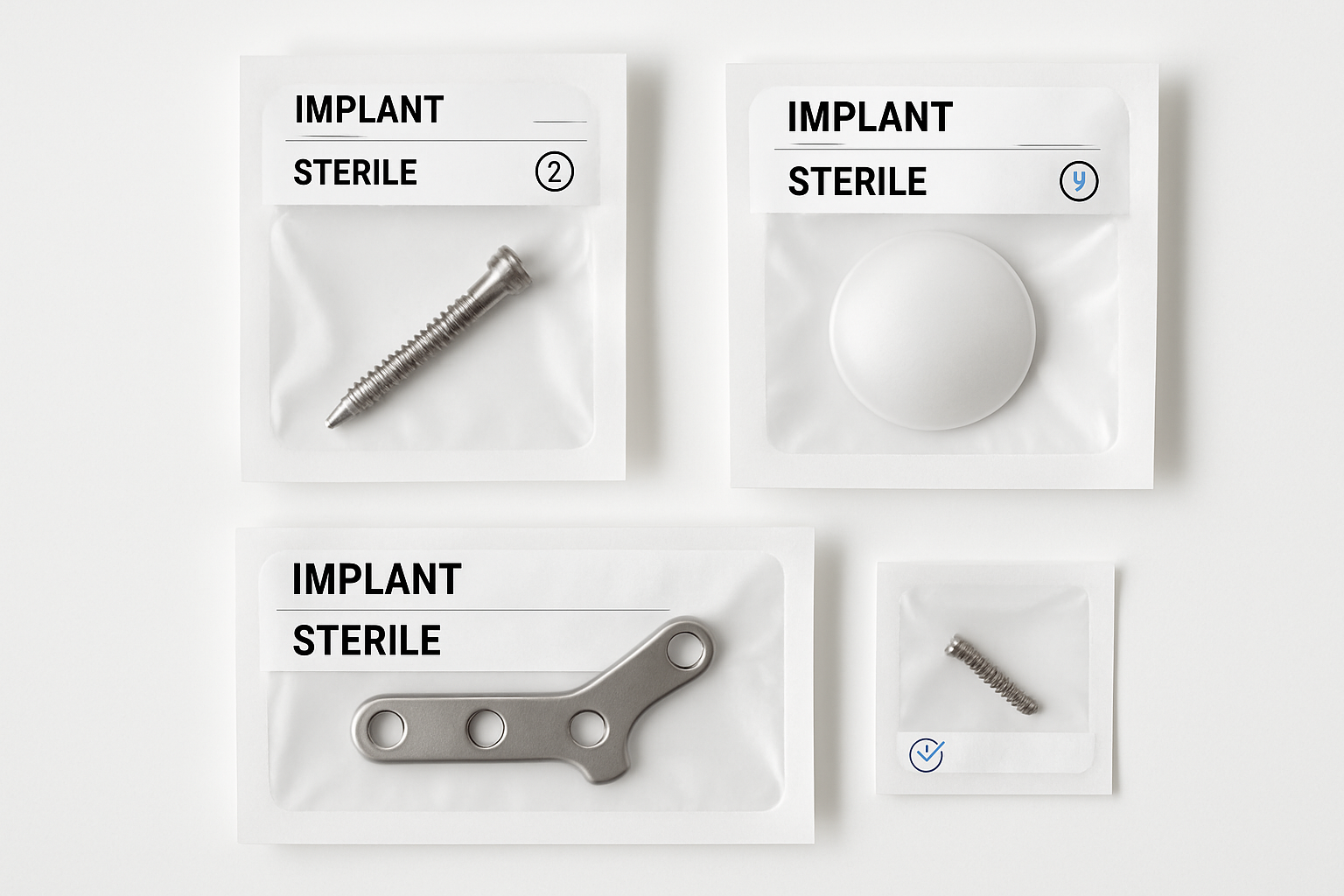
Controlled Assembly & Packaging
Our clean rooms support assembly, inspection, and packaging for sterile medical devices and sensitive aerospace components. Procedures follow ISO and FDA expectations with documented environmental monitoring and operator training.
- ISO-certified clean room environments with gowning protocols
- Lot traceability and device history records
- Sterile barrier packaging and labeling controls
- Final inspection with calibrated measurement tools
Clean Room Services
Precision handling for medical and aerospace assemblies
Kitting & Labeling
Device kitting, UDI labeling, and packaging validation per customer protocols.
Sterilization Readiness
Pre-sterilization cleaning, documentation, and coordination with sterilization providers.
Environmental Monitoring
Scheduled particle counts, airflow verification, and sanitation records maintained for audits.